As we reach the end of what has been the most challenging and difficult year so far, we want to take the time to celebrate the achievements of our Affiliated Companies.
Despite challenging times there have been some fantastic updates, and you can explore them using the dropdown boxes below. Click the logos at the top of each slide to go directly to each company website.
Abcam
New Directions in Immuno-oncology: Response and Resistance
Join us in February 2021 for live talks and discussions in the field of immuno-oncology
Abstract deadline: 4th January
Description:
Immuno-oncology, the approach of harnessing the immune system to help fight cancer, has emerged in the last decade as one of the fastest-growing areas in cancer research with hundreds of potential treatments under investigation. As the field has accelerated, the issue of immuno-oncology resistance has arisen and is now a key area of high unmet medical need for cancer researchers to understand and address. Join us in February for a series of interactive digital sessions dedicated to the latest breakthroughs in cell therapies, resistance to immunotherapies (tumor microenvironment and overcoming acquired resistance), and multiplex technologies. Gain insights into approaches impacting the field with talks from thought leaders across industry and academia, and opportunities to discuss key topics in live Q&A sessions.
Find out more:
Have a question? Please contact the Abcam Events Team.
ActiveMotif
Active Motif announced the launch of their new CUT&Tag-IT™ Assay Kit, the first and only complete kit for CUT&Tag experiments. CUT&Tag assays rapidly produce high-quality histone modification profiling and DNA-binding protein results on a genome-wide scale from less starting material than ChIP-Seq, and enables robust analysis from lower sequencing depths, saving both time and money.
Read more here, and contact Sarantis Chlamydas at chlamydas@activemotif.com
Aptamer Group
Aptamer Group advances COVID-19 testing capabilities with Integumen
Aptamer Group Ltd, the developer of diagnostic and therapeutic aptamer reagents, is pleased to announce further progress in their collaboration with Integumen plc (AIM: SKIN) to develop Microtox PD, a wastewater detection system for COVID-19. Aptamer Group is also collaborating with Integumen on the development of a new personalised rapid COVID-19 breath test, Microtx BT, and its complementary Digital Health Pass platform.
Both COVID-19 diagnostic platforms will incorporate Aptamer Group’s recently developed aptamers, highly specific to the SARS-CoV-2 spike protein, to detect the presence of the coronavirus and provide real-time detection of COVID-19.
COVID-19 has been associated with gastrointestinal symptoms in over 60% of positive patients and the SARS-COV-2 virus was found in their faecal samples. Sampling wastewater from households may therefore provide an early warning system for localised outbreaks in communities. Initial integration into the Microtox PD wastewater detection system and successful validation of the SARS-CoV-2 aptamer reagent for viral capture has been demonstrated. This detection system will now undergo full testing with real SARS-CoV-2 virus samples at the University of Aberdeen. It is anticipated that this detection system for the continuous detection of SARS-CoV-2 in sewage could be launched in early 2021 and will be distributed through Modern Water’s global footprint of over 3,000 installations.
Development of the Microtox BT breath test is intended to offer daily triaging for potential COVID-19 infection with a 24 hour Digital Health Pass that could allow the economy to re-open through personalised entry into specific social locations, such as airports, offices and events. This system uses Aptamer Group’s SARS-CoV-2 aptamers to offer rapid COVID-19 diagnosis via a simple, unobtrusive breath sample. Extensive first-round testing of this system has been successfully completed. Further testing on real COVID-19 virus samples is planned at the University of Aberdeen along with a joint trial of up to 5,000 participants in parallel with third parties using PCR and antigen COVID-19 diagnostic tests. Results are anticipated before the end of the year.
Upon successful completion of these evaluations, Aptamer Group will enter into an agreement with Integumen for the supply of commercial-scale quantities of the SARS-CoV-2 aptamer to commercialise their Microtox detection systems globally, providing identification of localised COVID-19 hotspots in wastewater and personalised real-time Breath Test and Digital Health Pass devices.
https://aptamergroup.com/aptamer-group-advances-covid-19-testing-capabilities-with-integumen/
BioAscent
BioAscent invests £1m to expand drug discovery service offering
BioAscent has further enhanced its comprehensive service offering in integrated drug discovery and compound management, with a £1m investment in new instrumentation and equipment. The investment follows consistent growth, particularly over the last two years, with numerous successful projects delivered by BioAscent’s growing team of highly experienced drug discovery scientists.
The additional instrumentation includes a FLIPR Penta screening system and a Biacore 8k Surface Plasmon Resonance (SPR) system, as well as a new workstation for plate set-up during compound management, and updated IT infrastructure.
The new FLIPR Penta screening system gives BioAscent additional capabilities in developing and running high-throughput cellular screening assays for measuring rapid kinetic events, such as intracellular ion flux in response to GPCR or ion channel activation. GPCRs are one of the most successful target classes for drug development, and BioAscent has proven expertise and a growing reputation in GPCR research. The company has recently conducted a number of successful GPCR hit-finding projects for biotech customers, and since the new system was installed, the team has already commenced a high throughput screen of the BioAscent 125k diversity library against a GPCR target.
The new Biacore 8k SPR system gives BioAscent a valuable orthogonal technology to validate clients’ hit compounds, as well as profile the kinetics of compounds in hit to lead and lead optimisation programmes. The technology also provides BioAscent with an additional key technique for screening its own internal fragment library for clients and complements the exceptional biophysical assay development and screening capabilities and expertise already offered via its existing Thermal Shift and Microscale Thermophoresis (MST) capabilities.
BioAscent’s compound management service continues to grow successfully, achieving compound annual revenue growth of 100% per year since 2016. The company offers state-of-the-art compound stores and manages customer libraries ranging from a few hundred to a few hundred thousand compounds, in both liquid and solid formats.
BioAscent’s Co-founder and Chief Operating Officer, Sylviane Boucharens, said: “We have always given customers the peace of mind that their compounds are stored securely, their inventory is up-to-date and trackable, and compounds can be accessed and delivered quickly and easily in screen-ready format – something that is especially critical for innovative biotechs working with a network of CROs. With the new equipment we can now offer even more sophisticated plating at a very cost-effective price, and the increased capacity will mean reduced lead times for clients.”
Commenting on the investment Paul Smith, BioAscent’s CEO, said: “We have the experience and expertise in the team to support a wide range of drug discovery projects, and we are committed to delivering the highest quality research, cost-effectively for our clients. Our investment in new systems and ensuring that the team has the best instrumentation at their disposal is a reflection of this commitment.”
Biomax
Biomax Informatics have released NICARA, a knowledge hub for neuroimaging data. NICARA stands for “NeuroImaging-based Connectome Assessment in Research and Application” and consists of mix-and-match modules for building, browsing, editing and comparing connectomes. In three webinars between October and December 2020, Biomax presented the applications of NICARA for clinicians, researchers and pharmacologists respectively.
More information is available at
https://www.biomax.com/product/nicara/
and on the Biomax YouTube channel:
Camena Bioscience
In February this year Camena Bioscience announced the achievement of another significant DNA synthesis technology milestone. Using gSynth, a novel multi-enzymatic de novo DNA synthesis and gene assembly technology, Camena Bioscience accurately produced a whole synthetic 2.7kb plasmid. Read more.
Founded in 2016, Camena Bioscience is a synthetic biology company that started in Cambridge and Pasadena. They’ve engineered new nucleic acid synthesis technology, which improves the quality and speed of synthesis. Our multi-enzymatic technology takes one approach to DNA, RNA and XNA synthesis, offering increased accuracy over existing Phosphoramidite synthesis technology.
Camena Bioscience aims to use their tools to enable the engineering of new synthetic biology applications.
If you’d like to collaborate please contact us now
Cytvia
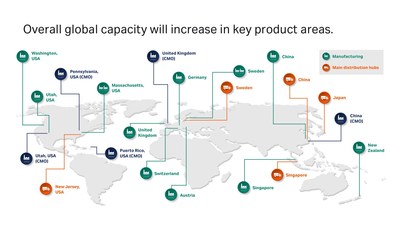
5 years, 500 million USD, and nearly 1,000 people: Cytiva invests for global capacity expansion
- Total planned investment is around 500 million USD over five years to raise manufacturing capacity
- Continues long-term strategy of increasing capacity to respond to growing industry demand and new market opportunities
- Cytiva hiring nearly 1,000 personnel in Austria, China, Singapore, Sweden, Switzerland, and the United States
- New manufacturing lines, 24/7 shift patterns, and increased automation will deliver additional manufacturing capacity
AMERSHAM, United Kingdom, Sept. 13, 2020 /PRNewswire/ — Cytiva, a global life sciences leader, is expanding its manufacturing capacity and hiring personnel in key areas to support the long-term growth of the biotechnology industry.
Emmanuel Ligner, President and CEO, Cytiva, says: “We know from our customers that availability and lead time are the most important considerations after quality. Cytiva’s long-term commitment is to deliver the best product, at the right time, and support our customers with expertise. The industry is growing rapidly, now even more due to COVID-19. Accelerating this investment will reinforce these commitments.”
While the COVID-19 pandemic is increasing short-term demand, the biotherapeutics industry was already predicted to grow by double digits between now and 20251. Cytiva’s global product manufacturing and distribution network encompasses 13 sites across Asia, the Americas, and Europe. The investments, totaling around 500 million USD, will respond to in-region, for-region demand, bolster security of supply through dual manufacturing, and increase overall global capacity in key product areas.
Cytiva is investing in talent, too, hiring nearly 1,000 people around the world. Ligner says: “We’re acquiring talent in every area of our business, from commercial to those on production lines, in order to better serve customers for the long term.”
Single-use technologies are used to manufacture 85%2 of the biologics currently in pre-commercial and clinical manufacturing lines. As regulatory approvals occur, demand for single-use products at manufacturing scale is expected to grow substantially. Through additional equipment and infrastructure at multiple sites, Cytiva’s capacity to manufacture single-use products will more than double.
In Asia-Pacific, single-use capacity will triple through a partnership with one of the largest healthcare technology suppliers in China, Wego, which is already producing consumables for Cytiva’s customers in the region.
Cell culture media production will increase thanks to new manufacturing lines and cleanroom space in Logan, Utah, as well as additional shifts and personnel. The Singapore and Pasching, Austria locations are increasing output through more personnel and additional work shifts. This follows on from a tenfold increase in powdered cell culture media production announced in May 2018.
The manufacturing capacity of Cytiva’s MabSelect and Capto chromatography product platforms has doubled, as part of a 70 million USD per year (2017 – 2022) capacity gains and facility modernization program at its Uppsala, Sweden site. Now, the plant is fully automated with the latest technology to allow continuous manufacturing. Other elements include the capacity extension of the Sephadex resin, setting-up additional facilities for in-house manufacturing, and the development of automation and digitalization infrastructure.
Cytiva is also enabling the rapidly growing cell and gene therapy market through an investment in a new facility in Grens, Switzerland to manufacture single-use kits for its Sepax and Sefia cell processing systems.
Cytiva has a longstanding and comprehensive Security of Supply program in place which enables manufacturing output to respond to market demands while ensuring that operations and service capabilities continue safely. For some product lines, part of the solution is having multiple sites able to deliver to customers.
Ligner says: “Dual manufacturing assures our customers that if one location encounters capacity constraints, we have plenty of back-up ready to activate.”
About Cytiva
Cytiva is a global life sciences leader with over 7,000 associates across 40 countries dedicated to advancing and accelerating therapeutics. As a trusted partner to customers that range in scale and scope, Cytiva brings speed, efficiency, and capacity to research and manufacturing workflows, enabling the development, manufacture, and delivery of transformative medicines to patients.
1 BioPlan’s 2020 Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, p 29
2 BioPlan’s 2020 Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, p 58
Definigen
19th November 2020, Cambridge: DefiniGEN – a provider of human cell products and services – has closed a £3.25 million funding round to accelerate its next phase of growth. This includes £2 million from BGF, the UK’s most active investor, alongside its existing consortium of investors led by 24Haymarket
DefiniGEN is a spin-out from the University of Cambridge, creating highly predictive disease cell models for preclinical research and drug development. The process of bringing new drugs to market is arduous, expensive and blighted by a high failure rate. DefiniGEN’s models help the biopharma industry to respond to these challenges, enabling highly relevant information on potential new drug efficacy to be realised in an accelerated manner.
The company’s unique technology platform achieves this using induced pluripotent stem cells (iPSCs), applying proprietary techniques to differentiate these into specific cell types and leveraging gene-editing tools to insert disease mutations.
DefiniGEN has capabilities across a range of cell types, with particular expertise in the liver disease field. Liver disease is currently ranked as the fifth most common cause of death in the UK and is receiving mounting interest in pharma R&D.
This funding will be used to boost DefiniGEN’s presence in the US, where the business has gained strong commercial traction. Investment will also be used to further expand DefiniGEN’s Cambridge facility and broaden its gene-editing capabilities.
Dr Marcus Yeo, co-founder and CEO of DefiniGEN, said: “Our technology platform harnesses iPSC technology to develop models that are transformational for drug discovery. We have gained significant commercial momentum in recent years, and this investment will be a significant driver of continued growth for DefiniGEN. We’re thrilled to be working with BGF, who understand the complex issues that we are addressing and are committed to helping us accelerate the next phase of growth.”
Lucy Edwardes-Jones, investor at BGF said: “DefiniGEN has a strong technology platform, differentiated IP, significant commercial validation and a platform that we believe is ready to support strong growth.
“From an investment perspective BGF has been active in supporting the UK’s life sciences industry, approaching 30 investments completed and £100m of capital deployed. DefiniGEN is a great example of a company that facilitates the process of discovering, developing and bringing much needed drugs to market. The market need is clearly emerging, and we believe there is a significant opportunity for DefiniGEN’s technology in the coming years.”
Paul Tselentis, CEO of 24Haymarket commented: “We are pleased to re-invest in DefiniGEN as the Company accelerates the commercialisation of its iPSC platform in the UK and internationally. We are excited to work alongside our new partners at BGF and welcome them onboard.”
Domainex
Forging a path of growth through the COVID-19 pandemic
Tom Mander, CEO of Domainex
Domainex, a leading provider of drug discovery services, has had a strong 2020 despite the COVID-19 pandemic. We are expecting ~40% growth in our revenues which is quite an achievement given the general economic woes many sectors have faced this year. This growth has been secured despite not being able to undertake the traditional mix of business development activities including travelling to visit clients and physically attend conferences or networking events. Instead, digital communications have come to the fore, including holding videocalls with prospects and communicating messaging digitally, via our website (www.domainex.co.uk) and our social media feeds. It’s led to a more efficient business development operation, saving resource and countless hours travelling.
At the outset of the pandemic our key consideration was how we could keep our staff safe while still serving our clients. We developed policies and procedures to cater for the impact of COVID-19 and have evolved these several times since as government guidelines have been updated. At the heart of our policy was a change in our working pattern: non-lab-based staff were asked to work from home while lab-based scientists have adopted a shift pattern as we moved to keeping our labs open 7 days/week, a practice that we are still following. I owe a big debt of gratitude to the fantastic team here at Domainex.
In order to support our growth, we’ve had to recruit. In total we have hired just under 30 scientists into Domainex this year – a nice blend of PhD/postdocs looking to use their skills in industry and seasoned industry scientists wanting to join us, attracted by the growth, our collaborative culture and our location in the Cambridge bioscience hub. Success in recruitment undoubtedly strengthens the company and positions us well to take on new projects in 2021. Fortunately, the greater utilisation of our facilities across the 7 days of operations has helped us to absorb this big new influx.
A lot of our growth is coming from overseas markets. We have been working with clients based across a number of countries in Western Europe, the United States and elsewhere including in Australia. Overall, we expect c. 50% of our revenues to come from overseas clients which will be a record for the company. On top of this we were able to complete our first out-licensing deal in the summer, to Invivoscribe Inc of San Diego, of a therapeutic candidate to treat multiple myeloma. Invivoscribe is now committed to taking the candidate drug into drug development; we stand to benefit through further milestone payments and potentially a royalty stream. Such a deal is a strong endorsement of the abilities of our scientists to invent new compounds with medicinal properties.
Interestingly, most of our growth has not been related to COVID-19. We are involved in one project with Sosie Heptares on Mpro, but most of our work has been across various other therapeutic areas. We are pleased to have received many positive comments back from our clients who have been very happy that we’ve been able to continue to support their drug discovery projects. We have also been nominated for further industry awards this year which is very humbling.
What of our future prospects? We plan to add further new hires to our ranks in the early part of 2021 and launch new services. We do expect to continue to operate our split-shift working pattern for the first few months of 2020. It will be a huge relief to eventually get back to working the way we were accustomed to before the pandemic. While some practices we’ll all be only too happy to see the back of, such as face mask wearing, undoubtedly some of our new practices will survive the end of the pandemic. We are cautiously optimistic that our business will continue to grow in 2021, with healthcare remaining very much in focus. Above all, we are looking forward to celebrating humanity, while being mindful of the many citizens who have lost their lives or livelihoods and the amazing sacrifices that so many on the front line have made. Never has the work we do in the life sciences felt quite as relevant as it does right now.
Genedata
Genedata Ltd Update – Significant growth in adopting Genedata software solutions in the UK
Genedata would like to thank all of its customers in Cambridge and across the UK who continue to rely on Genedata enterprise platforms to power their research and development.
Despite these challenging times, Genedata continues to innovate and rapidly expand its enterprise software platforms. The year 2020 saw further adoption of its software systems globally, particularly in the UK. E.g. Genedata Screener, the platform already extensively used within biopharma for small molecule screening, has been extended to cover complex large molecule screening workflows. The software has been used in several COVID-19 related projects in the UK, e.g. for COVID-19 PCR data analysis and COVID drug target screening. This has further cemented Genedata Screener as the globally market leading solution for biological screening to accelerate and streamline complex research processes.
We are also pleased to announce further significant developments this year with the deep-learning based Genedata Imagence platform which continues to provide real-world AI for high content screening and is being utilized increasingly for the most advanced imaging assay analysis. From us all at Genedata we wish you a happy, safe and prosperous 2021 and look forward to seeing you at our events in 2021.
If you would like to find out more about any of the Genedata platforms please visit the website: https://www.genedata.com
or email the team at uk@genedata.com
Genome Biologics
Genome Biologics was selected to join a new initiative by JLABS and BARDA, called Blue Knight™, where a limited number of hand-picked companies receive additional support for their technologies and approaches in the fight against COVID-19 and future pandemics – https://jlabs.jnjinnovation.com/blue-knight
This partnership was created between Johnson & Johnson Innovation and the Biomedical Advanced Research and Development Authority (BARDA), a component of the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health and Human Services. Blue Knight™ is a collaboration dedicated to anticipating potential health security threats, activating the global innovation community, and amplifying scientific and technological advancements with the aim to prepare for and respond to our rapidly evolving global health environment.
In addition, Genome Biologics entered into a research collaboration with AstraZeneca related to screening of proteins of the human secretome able to drive cardiac regeneration and recovery in Myocardial Infarction (MI), Heart Failure (HF) and Cardiotoxicity (CT).
Cardiovascular disease (CVD) remains the leading cause of death and disability globally, with WHO estimating 17.9 million lives are lost to CVD each year, with one third of these deaths occurring in people under 70 years of age and incurring more than $30B / year in healthcare related costs for individuals and governments.
This agreement with AstraZeneca Open Innovation will utilise our integrated discovery platform with our unique TrueCardium™ human heart organoids, with the potential to accelerate drug development and lead to new and innovative medicines to treat three very high unmet need areas of cardiovascular disease.
Healx
Healx, the AI-powered, patient-inspired technology company specialising in treatments for rare diseases, opened applications to v.2.0 of its Rare Treatment Accelerator in mid-November. Building on the success of their v.1.0 call last year, which was focused on patient group partnerships, Healx is now looking to build a global network of academic groups, patient groups and early-stage biotechs to accelerate promising drug repurposing opportunities to the clinic.
Recognising the all-too-common lack of financial investment and clinical expertise needed to translate repurposed drugs from research to reality, Healx is looking to partner with academic groups, patient groups and early-stage biotechs who have identified promising drug repurposing opportunities for rare diseases but need additional support to bring them to patients. Through the RTA, Healx will use its AI, drug development and clinical expertise, as well as significant financial resources, to evaluate, enhance and accelerate the repurposed drugs, with the aim of starting a clinical trial within 6 – 12 months.
The RTA 2.0 is open for applications now. For further information on the RTA or for FAQs, please click here.

Intellegens
Machine learning specialists Intellegens have teamed up with the drug discovery software experts from Optibrium to develop a new ‘deep learning’ platform tailored to small molecule drug discovery, Cerella™.
The platform is powered by Intellegens’ Alchemite™ technology, which extracts additional value from drug discovery data to make more accurate predictions, prioritise experimental efforts, and increase confidence in decisions; reducing costs whilst targeting high-quality compounds. The platform’s novel architecture combines on-premises deployment with cloud computing, providing both data security and scalability, and continuously learns from the latest data by directly connecting with a company’s data repository. The release of Cerella™ has been seen as a significant step that allowed validation of the underlying science through collaboration with some of the world’s biggest pharma. For Intellegens, which supports data-driven problem-solving across design and discovery, this is the first commercial partnership of its kind, extending the reach and availability of the Alchemite technology by embedding it in a partner’s platform.

Labkey
New and Intuitive Sample Management software that keeps up with your samples, and your science.
Complete laboratory sample management with sample tracking, data integration, and workflow tools.
Developed in collaboration with our lab partners, LabKey has successfully launched LabKey Sample Manager; a feature-rich and intuitive sample management software designed to boost the efficiency and productivity of your lab. Sample Manager allows users to easily track samples, manage and view their lineage and derivation, define laboratory workflows, and unify your samples with numerous laboratory instrument assay data.
Built on the foundation of the LabKey Server platform, Sample Manager can be augmented and configured to handle a variety of data management and analysis functions.
- End-to-end sample tracking features including chain-of-custody tracking, sample types and sources, and lineage views
- Integrate assay data with your samples and assign metadata for a complete picture of your ongoing experiments
- Assign samples to user-defined workflows and monitor the workflow completion status at each stage for every sample
NEW for January 2021:
- Freezer Management allowing users to locate samples in curated Freezers with check in / out and Freeze-Thaw cycle monitoring.
- Barcoding via an integration with BarTender, allowing users to seamlessly search and create sample labels
For more information, contact Jason Leadley on jasonl@labkey.com
Lifebit
Genomics England partners with Lifebit to power the UK Government’s COVID-19 research response
In a move to transform how genomics and clinical data is made usable, securely, for global biopharma and academic researchers, particularly in relation to COVID-19, Genomics England (GEL) has launched a groundbreaking Trusted Research Environment powered by Lifebit. This innovative federated research environment reflects a new chapter in the role that genomic research can play in collaborative drug discovery and treatment development to advance healthcare and patient outcomes.
Lifebit’s patented federated approach solves significant usability, scalability and security challenges. GEL users now have flexible research access to its growing, diverse data, enabling true collaboration across datasets and teams, all while guaranteeing Fort Knox-grade data security. Data stored on the platform never leaves GEL’s highly secure environment, but allows flexible research access to it. Researchers can introduce cutting edge analytical tools of their choice, link those tools securely with their in-house data, and conduct research in private workspaces that are audited, protected, and controlled by GEL’s strict information governance policies.
GEL CCO, Parker Moss, explains “The user’s external data does not move into GEL’s environment – it does not intermingle with our data and that’s great because it protects our data against malware. However, through federated links, you can research as if that data is in one place. It serves the joint benefit of not moving the data and also not putting our precious participants’ data at risk – that’s a very powerful value proposition”.
Learn more by watching Lifebit’s latest Population Genomics 2.0 webinar where Parker Moss shares insights and lessons learned from GEL’s federated research environment.
Microbiotica
Translating precision microbiome science into predictive biomarkers and more effective drugs
The microbiome is a multi-therapeutic opportunity that has the potential to revolutionize medicine. We know gut bacteria control disease and drug response throughout the body, and have evidence that modulating microbiota drives positive health outcomes. Yet, there remain technical barriers to predictable translation in this emerging field.
Built on over a decade of research in the Wellcome Sanger Institute, Microbiotica is addressing these barriers and helping to drive a new era of rigorous, data-driven microbiome R&D.
At Microbiotica, we have developed the ability to isolate the full complement of gut bacteria from patients, built the world’s leading Culture Collection and Reference Genome Database, and set up advanced bioinformatics and machine learning systems. Our capability in precisely characterising patient microbiota and linking them to patient phenotypes is unrivalled.
Armed with this industry-leading technology, we are working to discover bacterial biomarkers of drug response and best-in-class Live Bacterial Therapeutics (LBTs) in inflammatory bowel disease (IBD) and immuno-oncology (I-O), our current areas of therapeutic focus.
Our most recent development is in I-O, where we have a partnership with Cambridge University Hospitals and Cancer Research UK. Together we are tackling the link between the microbiome and response to checkpoint inhibitors, which has led to us now developing an LBT candidate that could improve response rates in patients.
With therapeutic candidates on the cusp of human testing, the Microbiotica team has moved into a new purpose-built facility in Cambridge this month. In these fresh new surroundings, we hope to be the focal point of a new, data-driven era of microbiome R&D, and be able to realise the full therapeutic potential of the microbiome field.
o2h
The mill scitech park – a lively culture for creators
o2h co-work labs is opening the doors to its incubator on the Mill SciTech park in April 2021. The o2h group, a Cambridge-based business with a vision to seed new ideas in life-science, tech and social enterprise acquired the 2.76 acre heritage Hauxton Mill site in 2018. The plans are to develop it into the Mill SciTech Park and shape one of the region’s most exciting community of entrepreneurs.
Prashant Shah, the Executive Chairman of the o2h group said that “the team at o2h ventures have developed a very close working relationship with the Milner Therapeutics Institute. We have been really impressed with their desire to work collaboratively with partners in the Cambridge area and they have championed some really groundbreaking ideas, events, companies and initiatives. We work together on seed funding some of the most interesting new ideas coming out of the UK and we look forward to building a deeper relationship with the launch of our incubator run by o2h co-work labs.”
A studio style workspace for life scientists and techies…
The o2h group has a passion for collaboration and the success of the mill scitech shall be dependent on the culture and community we create and nurture. The team driving o2h co-work labs wishes to create an intimate and close community of entrepreneurs, big companies, academics, and venture capitalists. We believe in the cross fertilisation of experiences and know-how across boundaries and want everyone in the community on the mill scitech park to know each other and contribute to each other’s success.
A sensitive heritage restoration in natural surroundings…
The mill which dates back to the 16th century was operating commercially as recently as 1972. The o2h group looks forward to lovingly renovating and restoring the old mill and inviting entrepreneurs to develop their breakthrough ideas in these unique surroundings. By popular demand from local residents, we will add a ground floor cafe in which they can mix with our resident entrepreneurs whilst looking over to the water flowing around the wheels. The site has a wide range of rare wildlife, ancient woodlands and a system of rivers and connecting brooks. The ecological studies shows the site supports water voles and otters as well as the maternity roosts for various rare species of bats.
A new era of science and technology…
Humanity is entering an unprecedented era of life science innovation allied with advancements in new technology such as AI is leading to the rapid discovery of new drug therapeutics. o2h ventures has been capitalising on these changes, being the first EIS knowledge intensive fund dedicated to investing in early stage biotech and therapeutic opportunities in the UK.
Join our community…
We have many ways in which the life science and tech community can contribute to the community at the Mill SciTech Park. As well as renting space in the incubator we have weekly on-site packages, and an associate membership to access hot desks, lab space and meeting rooms. We are also launching the UK’s first summer biotech bootcamp for experienced leaders from the field of life science.
For more details, please see – o2h.com/co-work-labs
Phoremost
PhoreMost, a drug discovery company based on the Babraham Research Campus, has had a very successful year despite the global pandemic, thanks in no small part to the strategy pioneered by its Senior Operations Director, Charli Howes and her team. The early introduction of measures such as antigen screening and obligatory masks enabled the 100% safe return of staff on-site, and the resumption of full productivity from August. PhoreMost’s pandemic operations manual has since been shared widely, to help other companies achieve the same outcome.
The year has seen two key appointments to PhoreMost’s leadership team, welcoming Dr Benedict Cross as CTO, and Dr Catherine Beech, OBE, to the Board as a Non-Executive Director. Ben has since been busy representing PhoreMost and presenting at various events – you can catch him next at the Virtual Protein Degradation & PROTAC Symposium in February 2021.
In the latter half of 2020, PhoreMost announced new collaboration agreements with XtalPi, an algorithm-driven artificial intelligence (AI)-based pharmaceutical technology company, and Oxford Biomedica, forming a strong Oxbridge partnership to develop next-generation CAR-T cell therapies with improved efficacy and durability. Both partnerships take advantage of PhoreMost’s unique SITESEEKER® platform, which exploits protein shape diversity to find new peptide targets, enhancing the power of phenotypic screening and translation into therapeutics.
Dr Chris Torrance, CEO of PhoreMost, said: “I would like to thank the whole team for adapting so well under some difficult circumstances this year, ensuring we were able to operate fully. We now look forward to the next exciting chapter in PhoreMost’s development, rapidly progressing our own internal portfolio of novel first-in-class targets, alongside working with partner organisations in increasingly diverse areas such as cell and gene therapies.”

Repure Life Science
Development of fibrosis therapy through the regulation of epigenetic regulatory enzymes of Repure Life Science
Fibrosis is an excessive formation of fibrous connective tissue in internal/externally stimulated organs or tissues, and fibrosis that makes up scar tissue, or accumulation of extracellular matrix molecules, is a common feature of chronic tissue damage. Lung, kidney, and liver fibrosis are one of the most common fibrotic diseases, and it accounts for up to 45% of all deaths in the United States.
Although fibrotic disease is not a rare disease, there is no clear cause and treatment method so far, so unmet medical demand is very high. In addition to idiopathic pulmonary fibrosis, chronic diseases such as diabetic nephropathy, chronic kidney failure, and cirrhosis of the liver are also affected by tissue fibrosis, resulting in fatal organ damage, leading to death. Because of this importance, new drugs targeting fibrosis-related diseases are designated as break through therapy by the US FDA and are rapidly approved.
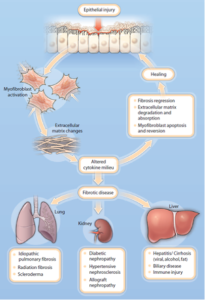
Figure 1.Common mechanisms of tissue fibrosis
Damage to epithelial cells, the cause of fibrosis, accumulates over time due to various genetic and environmental causes. These causes accumulate and cause abnormal expression changes of fibrotic genes when disease is induced, and epigenetic regulatory enzymes are involved. However, there have been many studies on gene expression according to pathological factors such as genetic profiling or diabetes/hypertension in fibrosis of specific tissues, but epigenetic control studies on the progression of fibrosis have hardly been conducted.
A representative epigenetic regulatory protein, Reversible transcriptional control between histone acetylation (HAT) and deacetylase (HDAC) is essential for balanced growth and division of cells, but dysfunction or absence of histone deacetylase is histone acetylation. By inducing excessive activation of the gene, abnormal gene expression is caused, which increases the likelihood of disease development.
Histone acetyltransferase enzyme regulates gene transcription by releasing condensed chromatin by attaching an acetyl group to a specific lysine residue in the histone tail, and post-translational modification of non-histone proteins, is known to play an important role in chronic intractable diseases such as cancer, inflammatory diseases, and brain diseases.
Actually, Repure Life Science, along with Yonsei university Medical school, has analyzed the clinical association of HAT proteins in various diseases through many years of research. In particular, p300 is used in idiopathic pulmonary fibrosis patients, kidney disease patients, NASH patients, and several animal models of fibrosis. The expression was greatly increased, and it was confirmed that fibrosis was effectively improved when targeting p300 at this time.
Based on this, a new compound (YRYP-1) that can specifically target p300 was developed, and this new compound showed the effect of effectively improving fibrosis by inhibiting the function of p300 through direct binding with p300.
Therefore, Repure Life Science proposes a new fibrosis treatment paradigm through the new p300 inhibitor YRYP-1, and aims to develop a fibrosis treatment through specific control of epigenetic modulators. It is preparing for clinical trials through a strategic alliance with Contract Research Organization (CRO) WuXi AppTec, which is capable of Contract Development Organization (CDO) / Contract Manufacturing Organization (CMO).
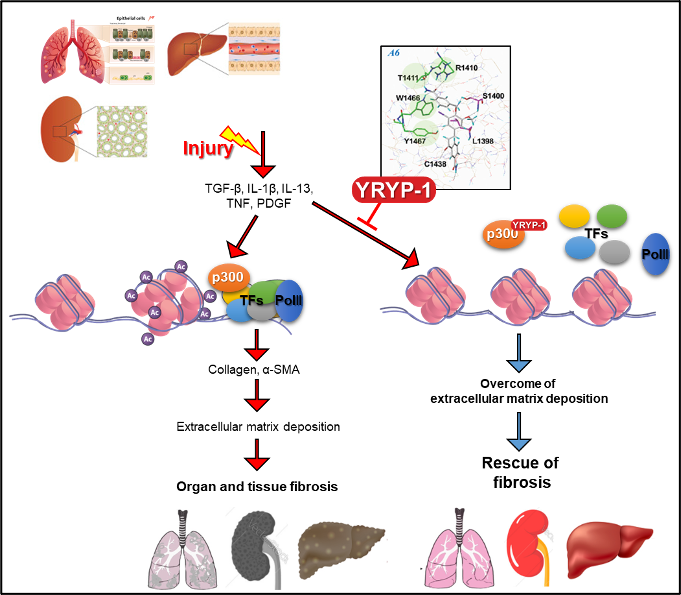
Figure 2 Mechanism of action of YRYP-1
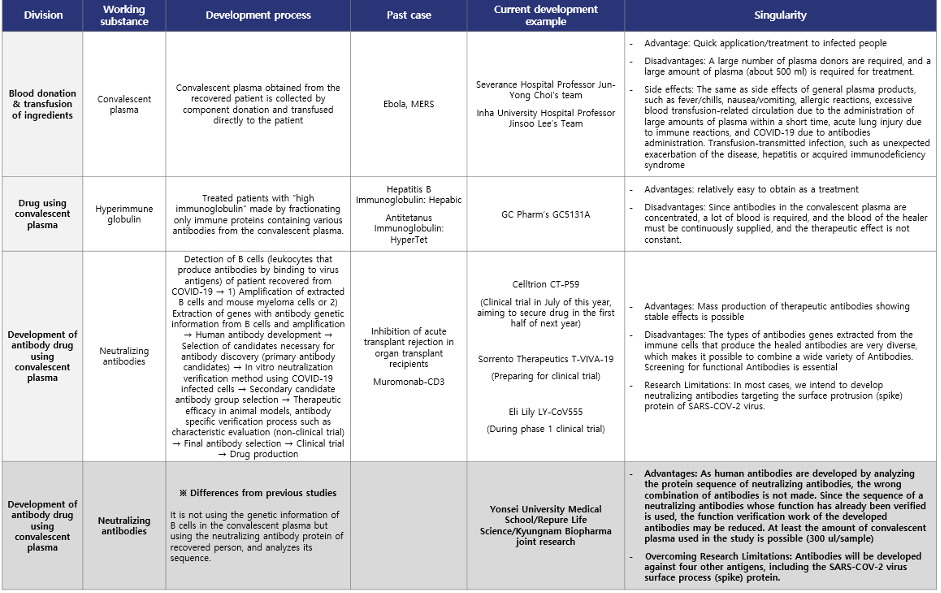
Repure Life Science/Yonsei University Severance Hospital Joint development of plasma-derived antibody therapeutics
On June 10th, Repure Life Science and Yonsei University Severance Hospital began joint research aimed at developing COVID-19 antibody drug. It is about differentiation from other COVID-19 drug.
In general, when developing antibody drug using convalescent plasma, B cells (white blood cells that produce antibodies) are detected in the convalescent plasma, and then genetic information that produces antibodies is extracted from them. Antibody candidates are selected based on the obtained genetic information, and neutralizing antibodies with high effectiveness are selected and secured through double neutralization testing. However, the antibody drug using convalescent plasma developed by Repure Life Science/Yonsei University Severance Hospital are primarily effective in neutralizing antibodies present in the convalescent plasma through a neutralizing ability test, and then the plasma with high neutralizing ability is selected. Then, the SARS-CoV-2 (coronavirus) antigen protein is used to obtain neutralizing antibodies by immunoprecipitation analysis. In addition, a differentiated strategy is taken to develop it as a COVID-19 antibody drug using convalescent plasma by securing the protein sequence of the obtained neutralizing antibodies via the high-tech proteomics analysis techniques.
Repure Life Science does not use only the spike protein of SARS-CoV-2 as an antigen, but with the envelope protein, membrane glycoprotein, nucleocapsid protein, and HE protein as antigens to detect neutralizing antibodies in plasma of recovered person from COVID-19. Therefore, there is a plan to develop a more effective antibody drug using convalescent plasma, leaving the possibility of detecting all neutralizing antibodies that can neutralize the coronavirus with another action as well as neutralizing antibodies targeting the virus’s spike protein. In addition, since antibody drug using convalescent plasma are being developed targeting all of the structural proteins of the virus, it is expected to be able to flexibly cope with various strains of coronavirus such as S, V, L, G, GH, and GR, so a wider range of patients infected with COVID-19 It is expected to be applicable.
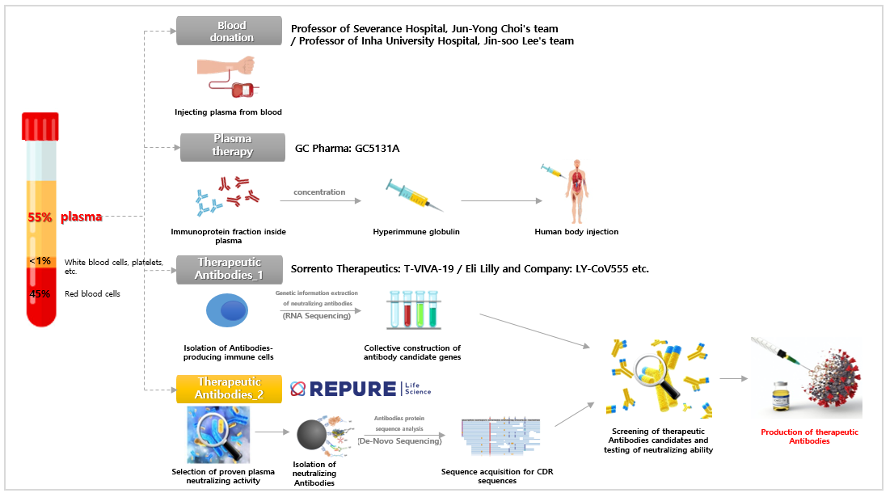
Developing therapeutic substances in a differentiated way from the existing one is a valuable research approach that can enable the development of new and flexible therapeutic agents for the outbreak of new infectious diseases in the future.
Table 1. Comparison of differences COVID-19 drug using convalescent plasma
RxCelerate
This was an exciting year for RxCelerate. In the summer, the Company moved its Cambridge HQ by taking 25,000 sq ft of prime office and lab space at BioMed Realty’s Dorothy Hodgkin building on the Babraham Research Campus. RxCelerate was one of the few companies in the Cambridge Cluster that kept its labs open to support clients in delivering their drug discovery programmes during the lockdown. RxCelerate has undergone rapid expansion over the last five years, adding significant new services to its out-sourced drug R&D platform and employing over 50 staff members.
The Company operates a unique model, providing capabilities to discover and develop drugs for clients, designing as well as executing every aspect of the research and development plan. The biology team specialises in in vivo pharmacology, offering proprietary models of a wide range of human diseases, as well as complex cell-based assays. The chemistry team provides medicinal chemistry, including in silico screening, and synthesis that integrates seamlessly with the in vitro testing capability. A dedicated team designs the optimum discovery or development strategy for each project and provides integrated program management. By working closely together, these teams can deliver clinical drug candidates at a fraction of the cost of traditional approaches. In addition, RxCelerate is very proud of its new in-house intravital microscopy (IVM) (https://www.rxcelerate.com/microscopy/) capability.
A virtual tour of RxCelerate’s new home is available here:
Selvita
Selvita to acquire Fidelta from Galapagos
Strengthening Selvita’s position as one of the largest preclinical contract research organizations in Europe.
Galapagos NV and Selvita S.A. announced on the 23rd of November that they have signed an agreement under which Selvita will acquire 100% of the outstanding shares in Fidelta d.o.o. for an enterprise value of € 31.2M plus the customary adjustments for net cash and working capital.
Selvita is a ‘one-stop shop’ CRO providing multidisciplinary support in resolving unique challenges of research within the area of drug discovery and development. The company currently employs over 550 professionals, of which over 1/3 hold a Ph.D. title. Selvita is headquartered in Krakow, Poland, with a second research site in Poznan, Poland, and foreign offices located in Cambridge, MA, and South San Francisco, in the U.S., as well as in Cambridge, UK.
Fidelta is a contract research organization within the Galapagos Group of companies, with core scientific competences in inflammation, fibrosis, and anti-infectives. It currently employs 181 employees, including over 150 highly experienced scientists, providing integrated drug discovery services in the biotech and pharmaceutical industry, with a proven track record of accomplished laboratory projects over many years. Fidelta is located in state-of-the art R&D facilities in Zagreb, Croatia, which offer almost 6,000 m2 of research space. The transaction is subject to customary closing conditions and is expected to close on the 4th of January 2021.
The scope of services provided by Fidelta is complementary to Selvita’s offerings and will enable Selvita to build a competitive advantage in business areas such as DMPK, in vivo pharmacology, and toxicology. Selvita will also increase its scale of operations within medicinal chemistry and in vitro pharmacology, resulting in a significant strengthening of its market position.
Fidelta’s therapeutic areas of expertise align with current market trends, as infectious diseases, inflammation, and fibrosis are all areas of core expertise for Fidelta and are becoming increasingly major areas of interest for pharma companies due to the significant and unmet medical needs.
More information: https://selvita.com/news/selvita-to-acquire-fidelta-from-galapagos/
Standigm
Standigm is a drug discovery company that searches therapeutic lead compounds using advanced artificial intelligence trained on biomedical big data.
Standigm has applied deep generative models to design novel therapeutic compounds and launched Standigm BEST®, a proprietary molecular generative platform for lead discovery and optimization in 2018. On top of the main molecular generative algorithm, we developed a proprietary system, MolFactory™ to automate molecule generation and data processing with scalable and distributed system architecture. It is the core of the Standigm’s molecular design workflow with minimal human input to prioritize and optimize machine generated compounds for further synthesis and experimental validation.It is being used to not only generate early virtual hit or lead compounds which are very novel and active to specific target protein, and of desirable drug-likeness but also further optimize potency and DMPK profiles. It also aids computational and medicinal chemists to get chemical insights with visual interpretation of the latent vector space which learned chemical space contexts such as structures and functions.
Standigm also developed iCLUE&ASK™, a target identification platform consisting of three components: a heterogeneous knowledge graph, an attention mechanism-aided deep neural network, and an interactive user interface. A deep neural network prioritizes disease targets based on the knowledge graph and the attention mechanism suggests supporting evidence for the prioritization. iCLUE&ASK™ provides an interactive user interface not only to report prediction results but to let users explore the underlying evidence.
Founded in 2015 by experts in artificial intelligence and systems biology at Samsung Advanced Institute of Technology, Standigm has grown into a team of 37 members including researchers (54% PhDs) of multi-disciplinary expertise in AI science and engineering, chemistry, biology and pharmacology. Standigm has raised a total of $22 million in funds including its current Series B funding. We will use the funds to scale the AI technology platforms and advance our drug discovery pipelines toward license-out. Our vision is a full-stack pharmaceutical company that could ease the pains of patients all over the world.
Storm Therapeutics
STORM Therapeutics selects first-in-class clinical candidate targeting METTL3
STORM Therapeutics, the leading biotechnology company focused on the discovery and development of small molecule therapies modulating RNA epigenetics, today announces that STC-15, its first-in-class drug candidate targeting METTL3, has been selected for development towards first in human clinical studies. The Company intends to submit an IND application in 2021.
STC-15 is an orally bioavailable, small molecule METTL3 inhibitor targeting an entirely new mechanism of action (modulation of RNA epigenetics) to treat acute myeloid leukaemia (AML) and other solid and haematological cancers.
Clinical candidate selection demonstrates STORM’s ground-breaking work on targeting RNA modifying enzymes for the development of new anti-cancer therapeutics. STORM has used state-of-the-art drug discovery capabilities, combined with unique analytical technologies specifically developed to target RNA epigenetics, to generate highly potent selective and orally bioavailable, small molecule inhibitors of METTL3 and other RNA modifying enzymes.
Keith Blundy, CEO of STORM Therapeutics, said: “STC-15, a highly potent and selective METTL3 inhibitor, is effective in leukaemia cells refractory to chemotherapy treatment. This patient population will be incorporated into the initial clinical trials aiming to accelerate clinical proof of concept for patients with limited other options in addition to exploring combinations with standard of care.
STORM leads the global field of RNA modulation having demonstrated in vivo proof of concept activity of the first RNA methyltransferase inhibitor in relevant animal models for myeloid and solid tumours. METTL3 is one of two programmes from the STORM platform to show in vivo activity, with others to follow.”
STORM showcased the activity of first-in-class METTL3 inhibitors in a range of solid tumours, (building on prior POC data in AML from 2019) at two scientific conferences in July 2020.
CONTACTS:
STORM Therapeutics Ltd
Keith Blundy
T: +44 (0)1223 804174
info@stormtherapeutics.com
Optimum Strategic Communications
Mary Clark, Eva Haas, Shabnam Bashir
Tel: +44 (0)203 950 9144
storm@optimumcomms.com
About STORM
STORM Therapeutics, founded in 2015, is a University of Cambridge spin-out translating the ground-breaking work of Professors Tony Kouzarides and Eric Miska in RNA epigenetics into the discovery of first-in-class drugs in oncology and other diseases. Storm is the leading company tackling disease through modulating RNA modifying enzymes and is developing a unique platform and pipeline to address these enzyme classes, including RNA methyltransferases.
STORM is backed by blue chip investors Cambridge Innovation Capital, M Ventures, Pfizer Ventures, Taiho Ventures LLC, Seroba Life Sciences and IP Group, who share the team’s ambitions to build a world-leading company in the field.
Totient
Totient Leverages Integral Molecular’s Membrane Proteome Array to Advance Cancer Therapeutic Discovery
Integral Molecular and Totient announce a partnership leveraging Integral Molecular’s Membrane Proteome Array (MPA) target identification technology to advance the discovery of antibodies for cancer therapy. Totient reconstructs antibodies from tertiary lymphoid structures (TLS), which are structures formed in tissues affected by autoimmunity, infections, and cancer, collected from patients experiencing exceptional immune responses. The MPA will pinpoint the binding targets for a panel of these antibodies from its array of 6,000 human membrane proteins. The agreement includes milestones paid to Integral Molecular upon successful antibody development goals. Read more »
Recent Updates:
Twist
Twist Bioscience’s Q4 earnings for 2020 exceeded analyst expectations. In this Writing the Future article, Emily Leproust, Twist Bioscience CEO, reflects on the success of Twist Bioscience since the company filed a successful IPO in 2018.

Emily Leproust:
“The Bills” (Bill Banyai and Bill Peck) and I founded Twist Bioscience in 2013 and, with help from ARCH Ventures, Illumina, and many other committed employees and investors, we went public on October 30, 2018. Two years later, in Twist Bioscience’s Q4 earnings, we have just reported record revenue for fiscal 2020 of $90.1 million.
While we are pleased to have generated such excellent growth for our investors, we are particularly proud of the synthetic biology advances we have delivered – let’s call that scientific equity.
Science has always been a team sport. When I look back on the past two years, I think about the many successful partnerships we have entered. For example, we worked with Arzeda, TeselaGen Biotechnology, and Labcyte to develop an advanced DNA assembly platform to encode Arzeda’s designed proteins, accelerating the process and reducing costs.
The coronavirus pandemic catalyzed new collaborations. Twist technology has helped the Vanderbilt Vaccine Center develop neutralizing antibodies to prevent and treat COVID-19. We have also worked with Saint Louis University to test monoclonal antibodies and VHH nanobodies against coronavirus. Those are just two examples.
Two years ago, our biopharma group was relatively quiet. But for our most recent fiscal year, as of September 30, we have signed 13 revenue-generating partnerships, 8 of which have milestones and/or royalties. NGS is a similar story. In 2018, it was a small part of our business. Now it’s almost half of our overall revenue.
Because Twist plays such a vital role in the synthetic biology universe, we have helped enable seminal advances: New strategies to surveil, test, and track COVID-19 and other infectious diseases; rapid antibody development through DARPA’s P3 program; and Neogene Therapeutics’ emerging T cell therapies against cancer to name a few.
All this has happened because the Twist team – now numbering 550 – has executed so brilliantly. Our people believe in our silicon DNA synthesis platform and have spread that message everywhere, disrupting markets, and advancing great science.
We are far from done. In the next few years, we look forward to ongoing growth in synthetic biology, NGS, and biopharma as we continue to execute on our roadmap for DNA data storage. Early efforts have stored the Convention on the Rights of the Child, the Netflix series Biohackers and other data on DNA. IARPA’s Molecular Information Storage program utilizes Twist as its DNA synthesis provider for its DNA Data Storage Project. We also just announced that Twist, Illumina, and Western Digital have formed an alliance with Microsoft to establish the foundations for a cost-effective commercial archival storage ecosystem for the explosive growth of digital data.
The financial and scientific equity we have built in the past two years is a powerful endorsement for our technology and our team. But the ultimate rewards are measured in impact. By accelerating efforts to contain COVD-19, treat cancer and other diseases, improve data storage and develop unique products for countless applications, we are realizing synthetic biology and genomics amazing potential and improving the quality of life for people around the world.
QKine
Qkine, with UK biotechnology companies Manchester BIOGEL and Cellesce, is developing fully synthetic animal-free scaffolds to overcome barriers in sustainable and scalable organoid culture, in a project funded by Innovate UK
Cardiff-based organoid company, Cellesce, peptide 3D scaffold specialists Manchester BIOGEL and complex protein manufacturer, Qkine, have been awarded Innovate UK Sustainable Innovation Funding to develop fully synthetic, chemically defined three-dimensional (3D) scaffolds that more accurately mimic the physiological environment in the human body and enable manufacture scale-up and improved reproducibility of patient-derived organoids.
Organoids are three-dimensional (3D) structures derived from stem cells that mimic mammalian organs. These have transformative potential as new platforms for faster drug discovery and better model systems for determining drug efficacy and toxicity, as well as pushing forwards basic biological understanding by more accurately replicating the responses seen in humans and reducing the need for animal use in research. However, existing methods for growing organoids rely predominantly on a 3D growth matrix extracted from mouse tumors to provide a supporting structure. This material is complex and poorly defined, leading to challenges with scale-up and limiting use in drug discovery platforms and other research applications.
This project seeks to address these issues by combining the existing technologies of Manchester BIOGEL’s tuneable peptide hydrogel scaffolds with Qkine’s optimized high-purity growth factors to build a new 3D cell culture scaffold that will mimic the natural environment of the body. Importantly, all the components will be chemically defined and animal product free, enabling greater experimental reproducibility. Working together with the leaders in patient-derived organoid scale-up, Cellesce, they will develop and tailor these new materials for scalable and reproducible organoid culture.
Commenting on the grant award, Professor Aline Miller, CEO of Manchester BIOGEL said:
I am very excited about this project – not only will we establish a new collaborative consortium, but we will also bring together our significant expertise to contribute to the development of an enabling platform technology with pressing scientific need, and with strong commercial potential.
A successful outcome from the collaboration will lead to the development of improved human cell-based models. This addresses key scientific challenges in the stem cell and drug discovery sector, reduces animal use in research, and strengthens UK life science manufacturing to provide a long-term sustainable return on investment for UK PLC.
Colorectal cancer organoids stained with Hoechst (blue/green) and Phalloidin (yellow/red). Images provided by Cellesce, copyright National Physical Laboratory.
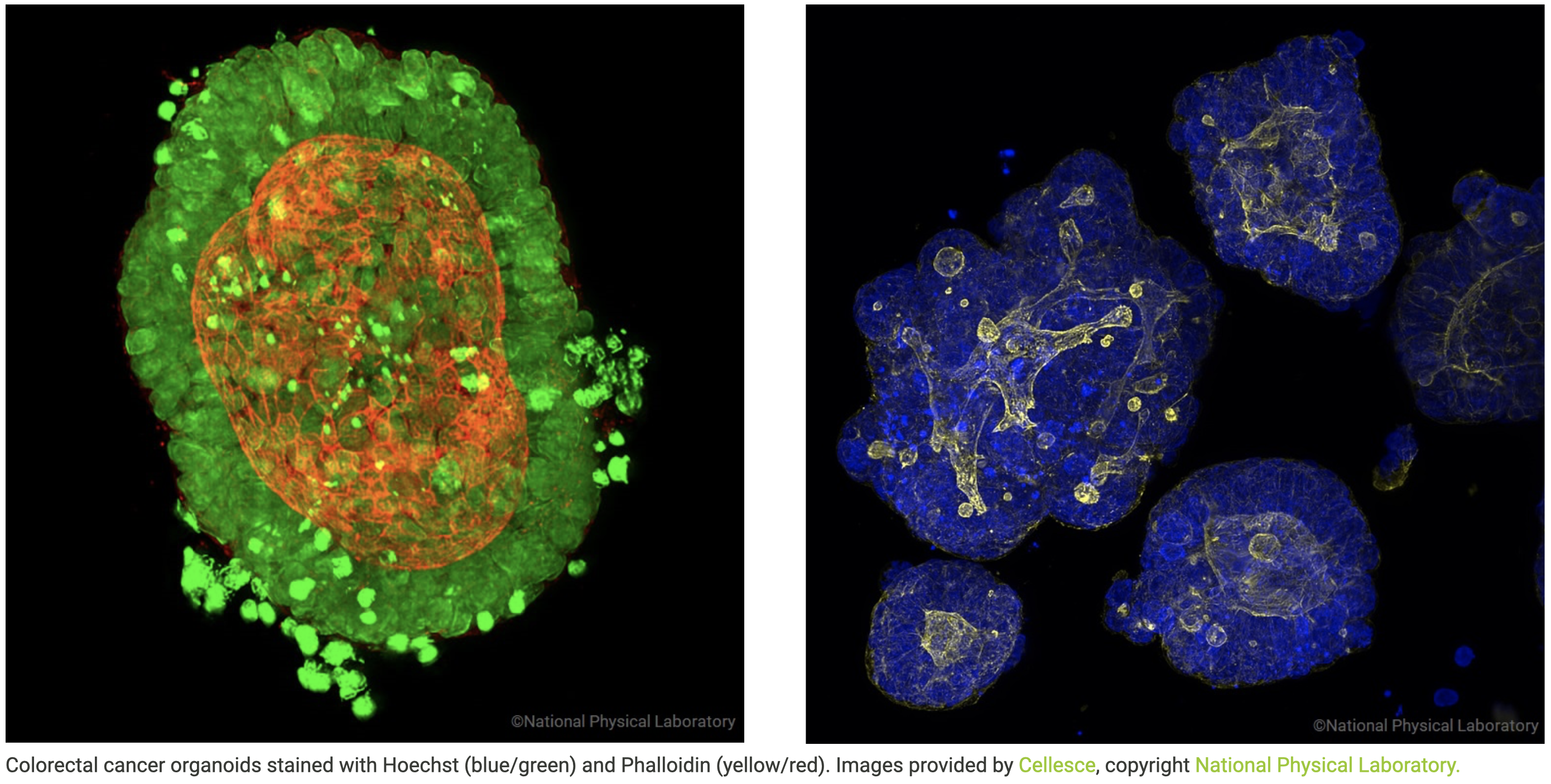
Cellesce is an early-stage life sciences company with key disruptive technology that enables more effective cancer drug discovery. Cellesce’s technology enables the growth and expansion at scale of organoids derived directly from patient tissue biopsies (Patient-Derived Organoids, PDOs). PDOs grow from adult stem cells and Cellesce uses them as better cancer models that more faithfully replicate and predict patient clinical responses to drug treatment. Cellesce provides PDO models to the pharma and biotech industry to drive improved cancer drug discovery, more products to market and better treatments for patients. The company’s goal is to provide a wide range of PDOs at unrivalled scale using its patented bioprocess technology, to enable efficient discovery and development of cancer therapies.
www.cellesce.com
Manchester BIOGEL is a global leader in the design and manufacture of synthetic self-assembling peptide hydrogels that provide a natural physiological extracellular matrix to support long term culture. Their biologically relevant hydrogels mimic the cell micro-environment and their stiffness and functionality can be modulated to simulate the natural environment of all human tissues. Manchester BIOGEL’s proprietary technology is 100% ethical, animal free and chemically defined. It opens up opportunities and offers clinically translatable solutions to meet current healthcare challenges within the growing fields of 3D cell culture, 3D bioprinting, tissue regeneration and drug discovery.
Virokine Therapeutics Ltd

Virokine Therapeutics Ltd is pleased to announce Virothera, which is dedicated to new immunotherapy for infectious disease. Covid reminded us this is a priority. Despite all the challenges, lab furloughs and very many zoom meetings, we are happy to report excellent preclinical in vivo results from our NIH sponsored study, with our first candidate, for STD, showing complete protection. Further therapeutics are in development, including for Covid. This PoC for our immunomodulator DNA approach was based on our new company patented platform technologies for antigen presentation and immunomodulation, which guide and focus the immune response to pathogens.
The incredible results for the first Covid RNA vaccines, highlighted the viability of the nucleic acid vaccine approach, and our novel technologies extend to both RNA and the more stable DNA. Virokine also welcomed distinguished new members to our team. Joining our Scientific advisory board, Professor E.S. Mocarski from the Emory Vaccine Centre and Stanford, is an expert in virus molecular pathogenesis. Joining as BD Director, Dr P. Hotten, former BD of Oxford Gene Technology and Royal Society Entrepreneur in Residence, is a BD start up and IP licencing specialist.
Into our next rounds, we are invited to Biotech Showcase as well as presenting at BioSeed, both new digital versions in January, so look forward to ‘seeing’ you there, not to mention the superb Milner symposia for the summer, maybe even progressing from virtual to reality!
For more information contact info@virokine.com